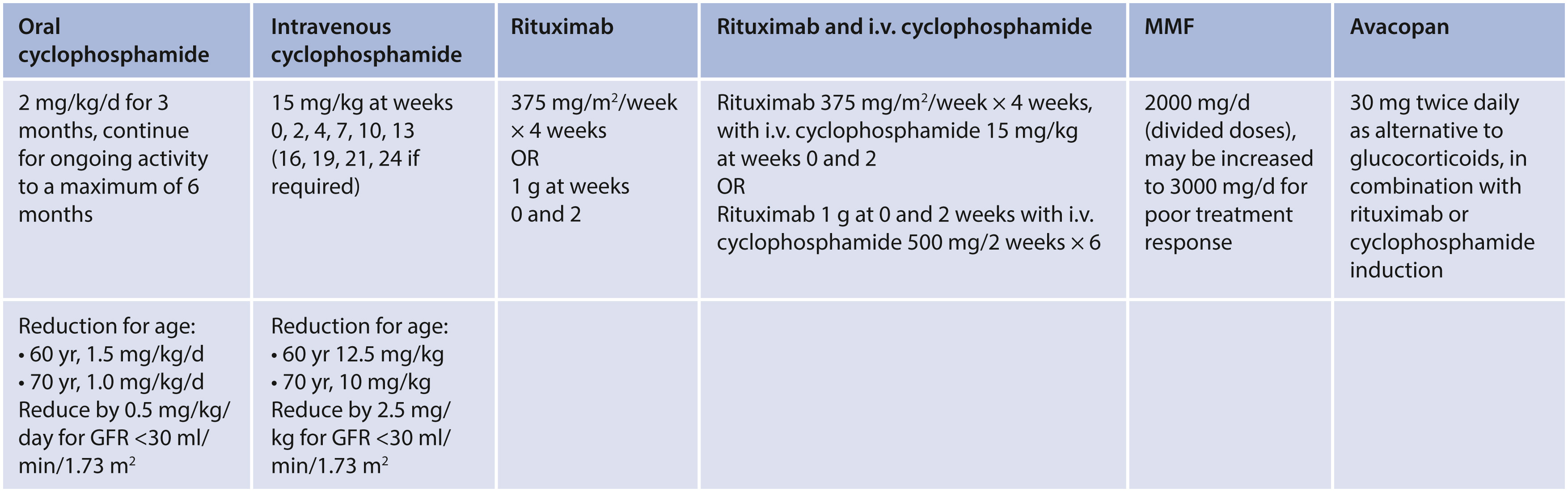
Maintanence
Remission: (BVAS=0, stable or improved eGFR, may have persistent urine changes)
Duration: 18 months – 4 years. Options: Ritux vs Aza (MMF) & pred wean
Ritux
- MAINRITSAN (vs aza – less major relapses, not minor) – 500mg*2 at remission, and 500mg at month 6, 12, 18.
- RITZAZREM (relapse, vs aza less major and minor) – 1g post induction, and month 4,8,12,16.
- Can dose as fixed or by CD19 cells ( same relapse rates, lower overall doses)
Aza
- 1.5-2mg/kg/day at remission for 1 year, then drop by 25mg every 3 months.
- 1.5-2mg/kg/day for 18-24 months, then drop 1mg/kg/day for 4 years, then taper 25mg every 3 months. Pred 5-7.5 for 2 years then drop 1mg/2 months.
- AZA-ANCA: 2 vs 4 years – relapse of 48 vs 24%
- MMF if intolerant. ( 1g bd for 2 years)
If still on HD at 3 months – consider discontinuation if no manifestations
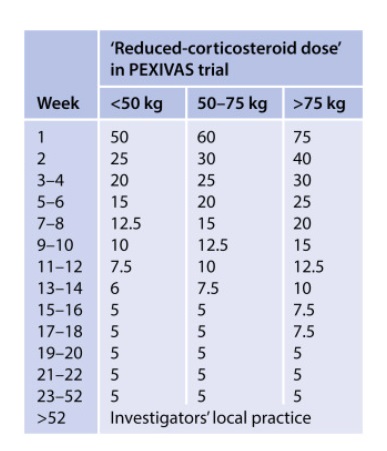
PEXIVAS taper ( as effective as high dose, less SE):