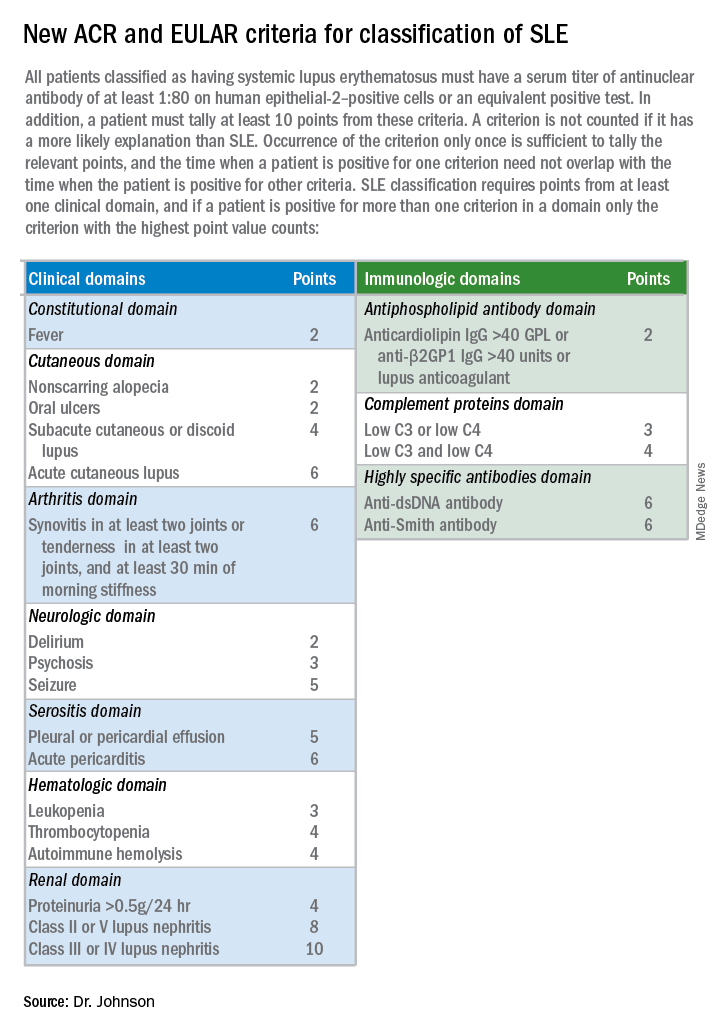
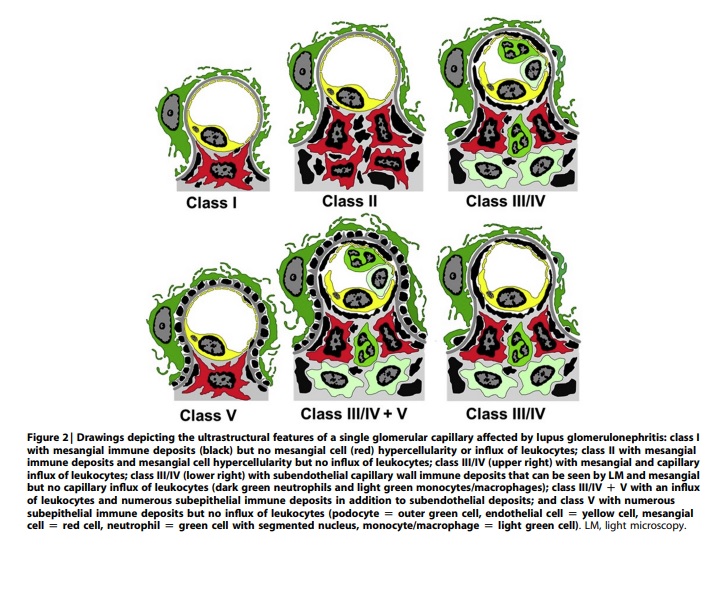
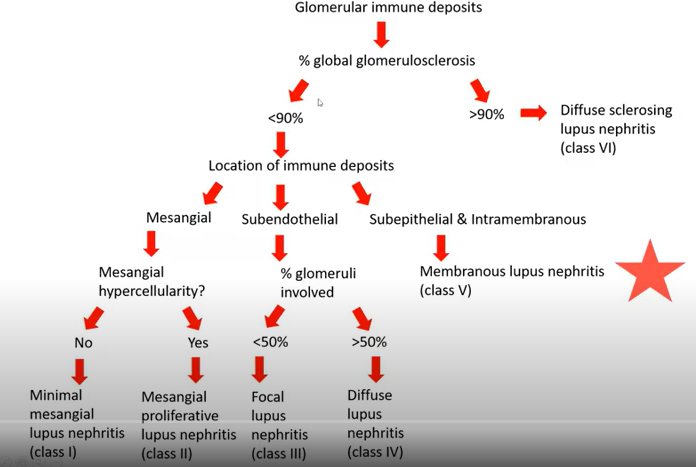
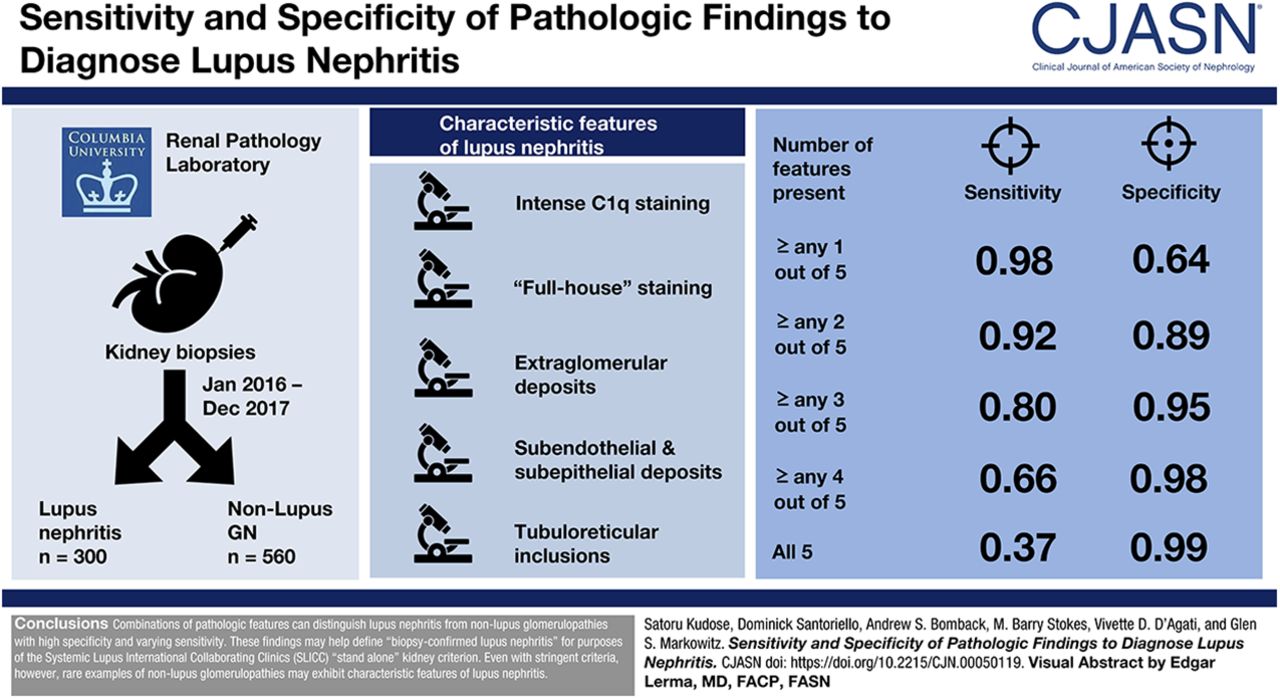
Induction
Severe proliferative (typically III and IV with or without a V
componentent)
- Steroids and either 1) MMF 2) CYC 3) Belimumab & MMF/low dose
CYC or 4) MMF and Tac (if eGFR >45)
Methylpred: 500-1000mg for 1-3 days, then oral pred
0.5-1mg/kg/day (ideal body weight) max 60mg taper over weeks to a
maintenance dose.
(Aurora-1): 25mg/day by day 3, wean to 2.5mg by week 16
(BLiss-LN): 25mg by week 7, 10mg by week 12
Plus CYC or MMF
- MMF 2-3g/day for 6 months
- IV CYC 500mg fortnightly for 3 months - 3 g total
(Eurolupus)
- IV CYC 0.5-1g/m2 q monthly * 6 (NIH protocol)
- PO CYC 1-1.5mg/kg/d, max 150mg/d for 2-4 months
Add on:
- Tac ( low dose -2mg bd - Chinese Data) &
MMF 500mg bd- if preserved function and intolerance of MMF +- diffuse
podocytopathy
- limited data on ritux but may have role if non
responsive/adherence
Considerations
- oral adherence required, CYC may be better in some cases
- CYC faster
Eurolupus
- Low dose vs NIH regime
- Follow up (5 years and 14 years)
- Equally well free of Renal flare/ ESRD
Criticism:
- mostly white northern European patients (however trials from SE asia
and abatacept trial had all patients on Eurolupus trial and no different
was observed suggesting this ethnicity isn’t a concern)
- MMF ( Dooley NEJM 2011) – suggests that MMF induction may be
inferior to CYC induction – slightly higher treatment failure ( but non
statistically significant)
KDIGO 21 Practice points
An MPAA-based regimen is the preferred initial therapy of
proliferative LN for patients at high risk of infertility, patients who
have a moderate to high prior cyclophosphamide exposure, and patients of
Asian, Hispanic, or African ancestry
triple immunosuppressive regimen that includes a CNI (tacrolimus
or cyclosporine) with reduced-dose MPAA and glucocorticoids is reserved
for patients who cannot tolerate standard-dose MPAA or are unfit for or
will not use cyclophosphamide-based regimens
In patients with baseline eGFR of at least 45 ml/min per 1.73 m2
, voclosporin can be added to MPAA and glucocorticoids as initial
therapy for 1 year
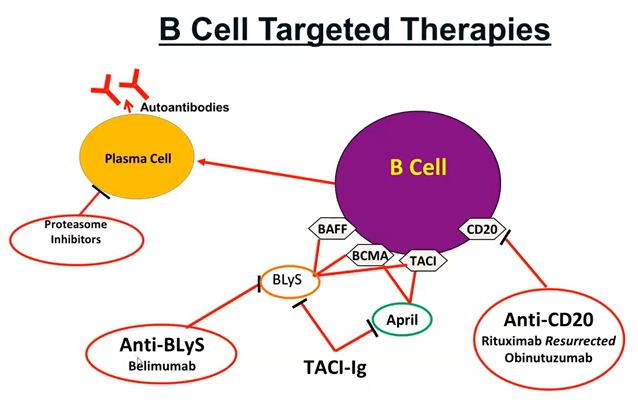
There is an emerging role for B lymphocyte targeting biologics in
the treatment of LN. Belimumab can be added to standard therapy in the
treatment of active LN. Rituximab may be considered for patients with
persistent disease activity or repeated flares
Maintenance
Pred taper as above - withdraw pred once sustained remission. Keep
maintainece dose <5 if required.
EULAR 2023 - 3 years IS total.
MMF 1-2g/day (first choice)
AZA 1-2.5mg/kg/d (pregnancy / intolerant of MMF)
CSA 2.5mg/kg/d/TAC (trough 4-6) if MMF/AZA not tolerated
discontinuation of glucocorticoids can be considered after
patients have maintained a complete clinical renal response for ‡12
months
total duration of initial immunosuppression plus combination
maintenance immunosuppression for proliferative LN should be over 36
months.
ALMS maintenance trial NEJM 2011
Time to treatment failure | n=227 | MMF vs AZA | European mild-mod
disease
Maintain nephritis ANN Rheum disease 2010
Time to renal flare | n= | MMF vs AZA |